Answer:
672 g
Step-by-step explanation:
We can calculate the mass of water that can be warmed from 25.0°C to 37.0°C by the addition of 8,064 calories using the following expression.
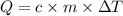
where,
c: specific heat of the water
m: mass
ΔT: change in the temperature
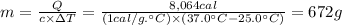
The mass of water that can be warmed under these conditions is 672 grams.