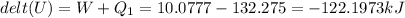Answer:
a) The work done is 10.0777 kJ
b) The water's change in internal energy is -122.1973 kJ
Step-by-step explanation:
Given data:
1 mol of liquid water
T₁ = temperature = 100.9°C
P = pressure = 1 atm
Endothermic reaction
T₂ = temperature = 100°C
1 mol of water vapor
VL = volume of liquid water = 18.8 mL = 0.0188 L
VG = volume of water vapor = 30.62 L
3.25 moles of liquid water vaporizes
Q = heat added to the system = -40.7 kJ
Questions: a) Calculate the work done on or by the system, W = ?
b) Calculate the water's change in internal energy, ΔU = ?
Heat for 3.25 moles:
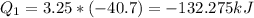
The work done:
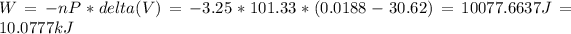
The change in internal energy: