It can be found that 337.5 g of AgCl formed from 100 g of silver nitrate and 258.4 g of AgCl from 100 g of CaCl₂.
Step-by-step explanation:
2AgNO₃ + CaCl₂ → 2 AgCl + Ca(NO₃)₂
We have to find the amount of AgCl formed from 100 g of Silver nitrate by writing the expression.
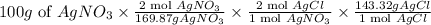
= 337.5 g AgCl
In the same way, we can find the amount of silver chloride produced from 100 g of Calcium chloride.
It can be found as 258.4 g of AgCl produced from 100 g of Calcium chloride.