246.94 litres is the volume is needed, if given 92.0 L of Hydrogen gas in a container of 612 kPa and reduces to 228kPa.
Step-by-step explanation:
Data given:
Initial volume of hydrogen gas in the container P1 = 92 litres
initial pressure of hydrogen gas in the container V1 = 612 kPa
final pressure of hydrogen gas on the container P2= 228kPa
final volume of hydrogen gas on the container V2 =?
from the data we will require equation of Boyle's law to calculate the unknown volume:
P1V1 = P2V2
V2 =
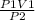
putting the values in the equation:
V2 =
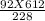
V2 = 246.94 Litres.
246.94 litres will be the volume of the hydrogen gas when pressure geta reduced to 228 kPa.