0.32 moles of Csl must be dissolved into 800 ml of a 0.40 M solution.
Step-by-step explanation:
Data given:
volume of the Csl solution = 800 ml or 0.8 litres
molarity of the solution = 0.40 M
number of moles of Csl = ?
molarity is calculated by using the formula:
molarity =
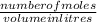
rearranging the equation to get the number of moles:
number of moles = molarity x volume in litres
putting the values in the equation:
number of moles = 0.4 x 0.8
number of moles of Csl in the solution is 0.32
therefore 0.32 moles of Csl should be dissolved in 800 ml of the solution to obtain molarity of 0.4 M.