1.22 litres will be the volume at a pressure of 1900 mm Hg if a gas occupies 3.06 liters at 760 mm Hg.
Step-by-step explanation:
Data given:
Initial volume of the gas V1 = 3.06 Litres
Initial pressure of the gas P1 = 760 mm Hg
final pressure of the gas P2 = 1900 m Hg
final volume of the gas V2 =?
From the data we can see that Boyle's Law will be applied here,
P1V1 = P2V2
Rearranging the equation, we get
V2 =
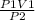
putting the values in the equation, we get
V2 =
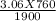
V2 = 1.224 Litres
The volume is 1.22 litres when the pressure is increased to 1900 mmHg.