Answer:
4,186 Joules of Heat Released
Step-by-step explanation:
When calculating Specific Heat, it is good to remember two formulas:
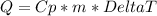
And
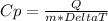
For this problem, we are using the first eqution to find the missing variable Q or the amount of joules released. The work would look like this:
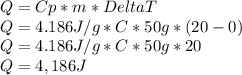
Hope this Helps!