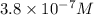Answer : The hydroxide ion concentration of a solution is,
Explanation :
As we know that:
pH : It is defined as the negative logarithm of hydrogen ion concentration.
Mathematically,
![pH=-\log [H^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/rjo2yhb5oj9ry1fr4db1ujrazm6fh3vhke.png)
Similarly,
pOH : It is defined as the negative logarithm of hydroxide ion concentration.
Mathematically,
![pOH=-\log [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/hdm1ob4dj6mx2sy3kobrrj91lzbh3927bk.png)
Given:
pOH = 6.42
![6.42=-\log [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/skuotucy7snb2ertknmmm6k2k9aoxzyk7r.png)
![[OH^-]=3.8* 10^(-7)M](https://img.qammunity.org/2021/formulas/chemistry/middle-school/mdxne5dcpcaki7ciqwo776enyl2i03z9ru.png)
Therefore, the hydroxide ion concentration of a solution is,