Answer : The number of moles of gas inside the car is, 105.0 mole.
Explanation :
To calculate the moles of gas we are using ideal gas equation.
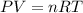
where,
P = Pressure of gas = 98.5 kPa = 0.972 atm (1 atm = 101.3 kPa)
V = Volume of gas = 2600 L
n = number of moles of gas = ?
R = Gas constant =
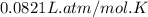
T = Temperature of gas =
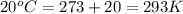
Now put all the given values in above equation, we get:
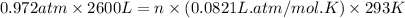
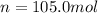
Therefore, the number of moles of gas inside the car is, 105.0 mole.