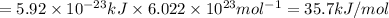Answer:
The difference in energy between the
 and
and
 states is 35.7 kJ/mol.
states is 35.7 kJ/mol.
Step-by-step explanation:
The Planck's equation :
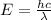
Where:
E = Energy of the electromagnetic radiations.
h = Planck's constant =
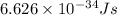
c = speed of light =
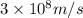
 = Wavelength of the electromagnetic radiations.
= Wavelength of the electromagnetic radiations.
Energy associated with
 transition :
transition :
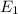
Wavelength associated with
 transition :
transition :
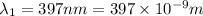
Energy associated with
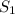 transition :
transition :
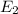
Wavelength associated with
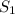 transition :
transition :
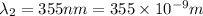
The difference in energy between the
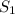 and
and
 states:
states:
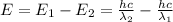
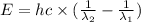
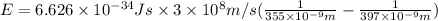
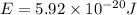
1 J = 0.001 kJ
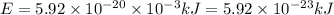
1 mole =

The difference in energy(kJ/mol) between the
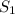 and
and
 states:
states: