Answer:
0.06375 moles
Step-by-step explanation:
Molarity is defined as moles of a solute per volume of solution.
-Given the molarity is 0.750m and the volume is 85ml
#Apply the molarity formula to solve for moles:
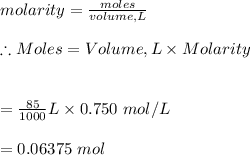
Hence, 0.06375 moles of sulfuric acid are needed.