Answer: New concentration of
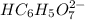 is 0.23 M.
is 0.23 M.
Step-by-step explanation:
The given data is as follows.
Moles of
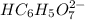 = 0.06 mol
= 0.06 mol
Moles of
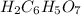 = 0.08 mol
= 0.08 mol
Therefore, moles of
 added are as follows.
added are as follows.
Moles of
 =
=
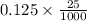
= 0.003125 mol
Now, new moles of
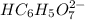 = 0.06 + 0.003125
= 0.06 + 0.003125
= 0.063125
Therefore, new concentration of
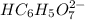 will be calculated as follows.
will be calculated as follows.
Concentration =
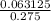
= 0.23 M
Thus, we can conclude that new concentration of
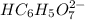 is 0.23 M.
is 0.23 M.