Answer : The molality of sodium chloride is, 1.79 m
Explanation :
To calculate the molality of solution, we use the equation:
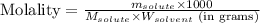
Where,
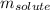 = Given mass of solute (sodium chloride) = 49 g
= Given mass of solute (sodium chloride) = 49 g
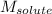 = Molar mass of solute (sodium chloride) = 58.5 g/mol
= Molar mass of solute (sodium chloride) = 58.5 g/mol
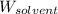 = Mass of solvent (water) = 469 g
= Mass of solvent (water) = 469 g
Now put all the given values in above equation, we get:
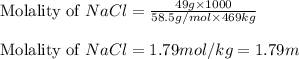
Hence, the molality of sodium chloride is, 1.79 m