Answer: Enthalpy for the dissolution reaction of one mole of aluminum nitrate is -158 kJ/mol.
Step-by-step explanation:
It is known that the density of water is 1 g/mL. So, mass of water will be calculated as follows.
Mass = volume × density
=
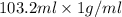
= 103.2 g
Specific heat of water is 4.18
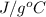 .
.
Now, we will calculate the enthalpy of solution as follows.
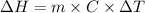
=
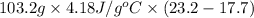
= 2372.5 J
As, 1 J =
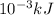 . So, 2372.5 J will be converted into kJ as follows.
. So, 2372.5 J will be converted into kJ as follows.
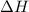 = 2.37 kJ
= 2.37 kJ
Molar mass of
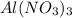 = 213 g/mol
= 213 g/mol
Hence, moles of
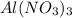 will be calculated as follows.
will be calculated as follows.
Moles of
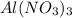 =
=
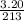
= 0.015
Therefore, enthalpy for the dissolution will be calculated as follows.
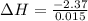
= -158 kJ/mol
Thus, we can conclude that enthalpy for the dissolution reaction of one mole of aluminum nitrate is -158 kJ/mol.