0.185 M is the molar concentration of sodium nitrate should appear on the label.
Step-by-step explanation:
Data given:
mass of NaNO3 = 3.166 grams
atomic mass of NaNO3 = 84.99 grams/mole
volume of the solution = 200 ml or 0.2 litres
molar concentration of sodium nitrate solution = ?
first number of moles of sodium nitrate is calculated as:
number of moles =

number of moles =
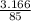
number of moles = 0.037 moles
molarity =
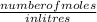
putting the values in the equation:
molarity =
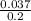
molarity = 0.185
Molar concentration of the sodium nitrate solution made with 3.166 grams of NaNO3 in 200 ml solution is 0.185 M