Answer:
The new pressure is 3.295 × 10⁵ Pa
Step-by-step explanation:
Given: Initial Volume: V₁ = 739 mL, Final Volume: V₂ = 244 mL,
At STP; Initial Temperature: T₁ = 273 K, Initial Pressure: P₁ = 10⁵ Pa,
Final Temperature: T₂ = 24°C = 24 + 273 = 297 K, (∵ 0°C = 273.15 K)
Final Pressure: P₂ = ?
According to the Combined Gas Law:
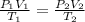
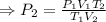
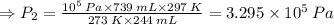
Therefore, the new or final pressure of the given gas: P₂ = 3.295 × 10⁵ Pa