Answer:
24 g
Step-by-step explanation:
To find the mass of precipitate that will be formed we need to use the solubility curve of KClO₃ in the water. From that graphic of solubility curve of KClO₃ in water, we have that:
The mass of the saturated solution of KClO₃ in 100 mL of water at 90 °C is = 50 g
The mass of the saturated solution of KClO₃ in 100 mL of water at 60 °C is = 26 g
Hence, by the difference of the mass of KClO₃ between 90 °C and 60 °C we can find the grams of precipitate that will be formed:
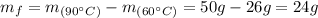
Therefore, 24 g of KClO₃ precipitate will be formed.
I hope it helps you!