Answer :
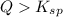 and a precipitate will form.
and a precipitate will form.
Explanation : Given,
Concentration of
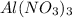 =
=
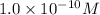
Concentration of
 =
=
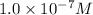
 of
of
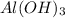 =
=
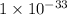
The equilibrium chemical reaction will be:
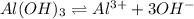
The expression of Q for this reaction is:
![Q=[Al^(3+)][OH^-]^3](https://img.qammunity.org/2021/formulas/chemistry/college/lo8ea98t3j7qgfhwvw0aya8rt8u97xolwf.png)
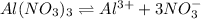
Concentration of
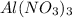 = Concentration of
= Concentration of
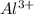 =
=
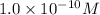
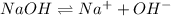
Concentration of
 = Concentration of
= Concentration of
 =
=
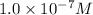
Now put all the given values in this expression, we get:
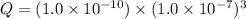
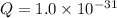
There are 3 conditions:
When
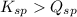 ; the reaction is product favored. (No precipitation)
; the reaction is product favored. (No precipitation)
When
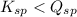 ; the reaction is reactant favored. (Precipitation)
; the reaction is reactant favored. (Precipitation)
When
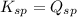 ; the reaction is in equilibrium. (Sparingly soluble)
; the reaction is in equilibrium. (Sparingly soluble)
As, the
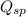 is more than
is more than
 . The above reaction is reactant favored. This means salt or precipitate will be formed.
. The above reaction is reactant favored. This means salt or precipitate will be formed.
Hence, the
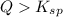 and a precipitate will form.
and a precipitate will form.