Answer : The number of moles of nitrogen present are, 0.737 mol
Explanation :
As we know that at STP, 1 mole of substance occupies 22.4 L volume of gas.
As per question,
As, 22.4 L volume of nitrogen gas present in 1 mole of nitrogen gas
Conversion used : 1 L = 1000 mL
Or, 22400 mL volume of nitrogen gas present in 1 mole of nitrogen gas
So, 16500 mL volume of nitrogen gas present in
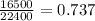 mole of nitrogen gas
mole of nitrogen gas
Therefore, the number of moles of nitrogen present are, 0.737 mol