Answer: (a) The solubility of CuCl in pure water is
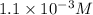 .
.
(b) The solubility of CuCl in 0.1 M NaCl is
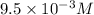 .
.
Step-by-step explanation:
(a) Chemical equation for the given reaction in pure water is as follows.
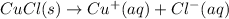
Initial: 0 0
Change: +x +x
Equilibm: x x
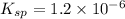
And, equilibrium expression is as follows.
![K_(sp) = [Cu^(+)][Cl^(-)]](https://img.qammunity.org/2021/formulas/chemistry/college/2vld1x5952ba4h3z4y63704j1w1l8zpu9q.png)
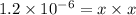
x =
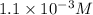
Hence, the solubility of CuCl in pure water is
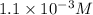 .
.
(b) When NaCl is 0.1 M,
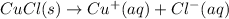 ,
,
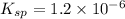
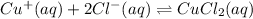 ,
,
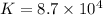
Net equation:
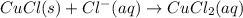
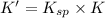
= 0.1044
So for,
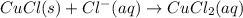
Initial: 0.1 0
Change: -x +x
Equilibm: 0.1 - x x
Now, the equilibrium expression is as follows.
K' =
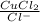
0.1044 =
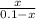
x =
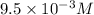
Therefore, the solubility of CuCl in 0.1 M NaCl is
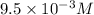 .
.