Answer:
The partial pressure of the gas produced by the reaction is 0.9187 atm
Step-by-step explanation:
Given;
vapor pressure of water,
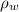 = 0.0313 atm
= 0.0313 atm
measured atmospheric pressure,
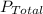 = 0.950 atm
= 0.950 atm
partial pressure of the gas produced by the reaction = ?
According to Dalton's law of partial pressure; total pressure is equal to sum of partial pressure of water vapor and gas produced.
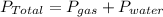
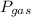 =
=
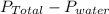
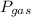 = 0.95 - 0.0313
= 0.95 - 0.0313
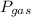 = 0.9187 atm
= 0.9187 atm
Therefore, the partial pressure of the gas produced by the reaction is 0.9187 atm