Answer: Partial pressure of
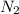 at a depth of 132 ft below sea level is 2964 mm Hg.
at a depth of 132 ft below sea level is 2964 mm Hg.
Step-by-step explanation:
It is known that 1 atm = 760 mm Hg.
Also,
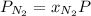
where,
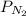 = partial pressure of
= partial pressure of
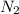
P = atmospheric pressure
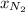 = mole fraction of
= mole fraction of
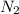
Putting the given values into the above formula as follows.
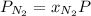
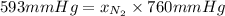
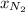 = 0.780
= 0.780
Now, at a depth of 132 ft below the surface of the water where pressure is 5.0 atm. So, partial pressure of
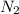 is as follows.
is as follows.
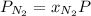
=
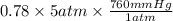
= 2964 mm Hg
Therefore, we can conclude that partial pressure of
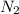 at a depth of 132 ft below sea level is 2964 mm Hg.
at a depth of 132 ft below sea level is 2964 mm Hg.