Answer : The correct option is, (C) 32.0 mL
Explanation :
Formula used :
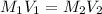
where,
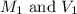 are the initial molarity and volume of NH₃.
are the initial molarity and volume of NH₃.
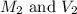 are the final molarity and volume of HCl.
are the final molarity and volume of HCl.
We are given:
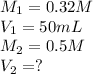
Now put all the given values in above equation, we get:
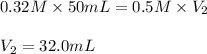
Hence, the volume of hydrochloric acid added to react completely with the ammonia must be, 32.0 mL