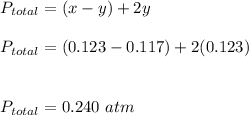Answer:
At
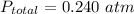 ; 95.0% of the molecules will dissociate at this temperature
; 95.0% of the molecules will dissociate at this temperature
Step-by-step explanation:
The chemical reaction of this dissociation is:
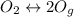
The ICE table is as follows:
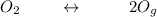
Initial 100 0
Change -83 +166
Equilibrium 17 166
The mole fractions of each constituent is now calculated as:
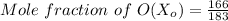 = 0.9071
= 0.9071
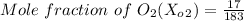 = 0.0929
= 0.0929
Given that the total pressure
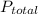 = 1.000 atm ; the partial pressure of each gas is calculated by using Raoult's Law.
= 1.000 atm ; the partial pressure of each gas is calculated by using Raoult's Law.
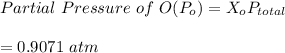
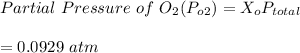
Now; we proceed to determine the equilibrium constant
 ; which is illustrated as:
; which is illustrated as:
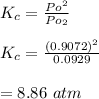
Let assume that the partial pressure of
 be x ;&
be x ;&
the change in pressure of
 be y ; then
be y ; then
we can write that the following as the changes in concentration of species :
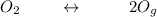
Initial x 0
Change -y +2 y
Equilibrium x - y 2 y
From above; we can rewrite our equilibrium constant as:

From the question; we are told that provided that 95% of the molecules dissociate at this temperature. Therefore, we have:
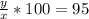 % -------- equation (2)
% -------- equation (2)
Solving and equating equation 1 and 2 ;
x = 0.123 atm
y = 0.117 atm
Thus, the pressure required can be calculated as :