Answer:
- Volume of the solution will increase.
- Molarity will decrease.
Step-by-step explanation:
Hello,
In this case, for molarity, unit of concentration relating the moles of solute and volume of solution, we have:
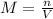
Hence, since the given molarity is 0.70M, we first shall infer that by adding more water, that for sure is the solvent, the volume of the solution will increase which will be the first change. Moreover, since the volume and the molarity are in an inversely proportional relationship, as the volume increases the molarity decreases, which is the other change. In addition to the aforementioned statements, the moles of the solute are not modified as just water is added.
Best regards.