119.91 litres is the new volume when a balloon with a volume of 30.0L at a pressure of 101.3 kPa rises to an altitude with a pressure of 25.3 kPa.
Step-by-step explanation:
Data given:
Initial volume of the balloon, V1 = 30 litres
Initial pressure on the balloon, P1 = 101.1.3 kPa or 0.998 atm
final pressure on the balloon, P2 = 25.3 Kpa or 0.24 atm
final volume of the balloon, V2
from the data provided, we must apply Boyle's Law to know the final volume of the balloon.
formula used:
P1V1 = P2V2
Rearranging the equation:
V2 =
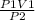
putting the values in the equation:
V2 =
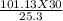
V2 = 119.91 litres.
We can see that when pressure was reduced to 25.3 kPa from 101.13 kPa, the volume increases to 119.91 litres.