Answer:
D) Benzene will not dissolve well in water since non-polar molecules do not dissolve in polar solvents.
Step-by-step explanation:
Benzene is nonpolar because it is a symmetrical molecule in which all the bond dipoles cancel.
Polar solvents have large dipole moments or partial charge; they contain bonds between atoms with very different electronegativities.
Polar solvents like water cannot dissolve non-polar substances like benzene.
Benzene can only be dissolved by other non-polar solvents like n-hexane, carbon tetrachloride
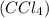 , e.t.c.
, e.t.c.