Answer:
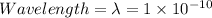 m
m
Explanation:
Given that the wavelengths for X-rays with frequency
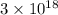
To find the wavelengths for the given X-rays:
The distance between two peaks in a wave is called the wavelength.
The value of wavelength is equal to the wave velocity divided by the frequency.
That is written by
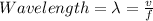
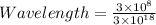
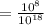
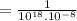 (by using the property
(by using the property
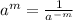 )
)
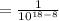 (by using the property
(by using the property
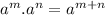 )
)
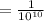
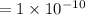 m (by using the property
m (by using the property
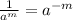 )
)
∴
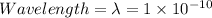 m
m