Sample C has the lowest kinetic energy value and, assuming the same conditions for all samples, it would also have the lowest temperature. Therefore, option C is correct
The image contains a list of samples with their corresponding average kinetic energies of molecules in joules. To determine which sample has the lowest temperature, we would need to apply the principle that, at a given temperature, the average kinetic energy of molecules is proportional to the temperature itself. This is described by the equation:
![\[ KE_(avg) = (3)/(2) k T \]](https://img.qammunity.org/2021/formulas/physics/middle-school/l4ln4rimy7g5sk5y83f87wn2ag8vk4mmpy.png)
where
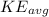 is the average kinetic energy,
is the average kinetic energy,
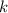 is the Boltzmann constant
is the Boltzmann constant
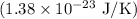 , and
, and
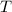 is the temperature in kelvins.
is the temperature in kelvins.
However, without additional information about the specific gases or the number of molecules involved, we cannot calculate the exact temperature from the average kinetic energy. Typically, the kinetic energy of a sample is directly proportional to its temperature (in an ideal gas scenario). Therefore, the sample with the lowest average kinetic energy would also have the lowest temperature.
Based on the average kinetic energy values provided in the image, we can conclude that:
- Sample A has
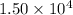 J
J
- Sample B has
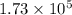 J
J
- Sample C has
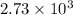 J
J
- Sample D has
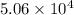 J
J
Sample C has the lowest kinetic energy value and, assuming the same conditions for all samples, it would also have the lowest temperature.