Answer:
The heat of vaporization of octane is 39.01KJ/mol.
Step-by-step explanation:
No chemical reaction take place without heat,because it is necessary for the formation of breaking of chemical bond.The reaction which does not require external energy is called as spontaneous energy and that which require external energy is called as non spontaneous energy.
By using Clausius - Clapeyron equation,
log(P₂/P₁) = Δh/2.303R(1/T₁- 1/T₂) eqn 1
P₁ is vapor pressure at temperature T₁
P₂ is vapor pressure at temperature T₂
Δh is heat of vaporization to find
P₁ = 40 Torr, T₁ =45.1°c = 318.25K
P₂ = 400 Torr, T₂ = 104.0°c. = 377.15K
substituting all values in eqn 1
Δh =
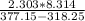 *(377.15*318.25
*(377.15*318.25
Δh = 39018.55J/mol
The heat of vaporization of octane is 39.01KJ/mol.