Answer:
Methane is produced as a new product.
Step-by-step explanation:
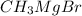 acts as a base toward -COOH group in p-methylbenzoic acid.
acts as a base toward -COOH group in p-methylbenzoic acid.
Hence an acid-base reaction occurs between p-methylbenzoic acid and
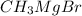 to produce methane and p-methylbenzoate.
to produce methane and p-methylbenzoate.
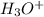 addition will convert p-methylbenzoate back to p-methylbenzoic acid.
addition will convert p-methylbenzoate back to p-methylbenzoic acid.
Hence, methane is produce as a new product.
Reaction sequences are given below.