100.4 degree celsius is the temperature of the water having non-electrolyte.
The correct answer is b.
Step-by-step explanation:
Data given:
mass of non-electrolyte solute = 31.65 grams
volume of water = 220 ml or 0.22 litres
molar mass of solute = 180.18 grams/mole
density of water = 1 gm/mole
Boiling point of water = 100 degrees celsius
molality = ?
Kb for water = 0.51
boiling point elevation or temperature of hot water,T =?
Formula used:
T = mKb equation 1
number of moles of non electrolyte =
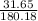
number of moles = 0.17 moles
molality =
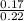
molality = 0.77 M
putting the values in equation 1
T = 0.77 X 0.51
T = 0.392 degree celsius. is the elevation in temperature when solute is added.
Temperature of the hot water = 100 +0.392
= 100.392 degree celsius.