Answer:
58.72 mL
Step-by-step explanation:
The chemical equation for the neutralization reaction is :
H₂SO₄(aq) + Na₂CO₃(s) --------------> Na₂SO₄(aq) + H₂O(l) + CO₂(g)
where;
M₁ = Molarity of H₂SO₄
M₂= Molarity of Na₂CO₃
V₁= Volume of H₂SO₄
V₂ = Volume of Na₂CO₃
Given that :
M₁ = 18.4 M
V₁= 0.3 mL
10% Na₂CO₃ means 100 g of solution contain 10 g of Na₂CO₃
i.e. 10 g Na₂CO₃ dissolved and diluted to 100 mL water.
Molar mass of Na₂CO₃ = 106 g/mol
106 g Na₂CO₃ dissolved in 100 mL will give 0.1 M Na₂CO₃ solution.
However;
If, 106 g Na₂CO₃ ≡ 0.1 M Na₂CO₃
Then, 10 g Na₂CO₃ ≡ 'A' M of Na₂CO₃
By cross multiplying; we have:
106 × A = 10 × 0.1
106 × A = 1
A = (1/106) M/100 mL
A = 10 x (1/106)) M/L
A = (10/106) M
A = 0.094 M
Therefore,the molarity of 10% Na₂CO₃ solution is 0.094 M.
For the Neutralization equation, we have:
M₁V₁ = M₂V₂
18.4×0.3 = 0.094×V₂
Making V₂ the subject of the formula;we have:
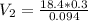
V₂ = 58.72 mL