Answer : The volume of nitrogen gas equal to
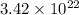 molecules of this substance is, 1.27 liters.
molecules of this substance is, 1.27 liters.
Explanation :
First we have to calculate the moles of nitrogen gas.
As,
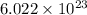 molecules present in 1 mole of nitrogen gas
molecules present in 1 mole of nitrogen gas
So,
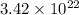 molecules present in
molecules present in
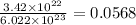 mole of nitrogen gas
mole of nitrogen gas
Now we have to calculate the volume of nitrogen gas.
As we know that, 1 mole of substance occupies 22.4 L volume of gas at STP.
As, 1 mole of nitrogen gas occupies 22.4 L volume of nitrogen gas
So, 0.0568 mole of nitrogen gas occupies
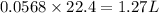 volume of nitrogen gas
volume of nitrogen gas
Therefore, the volume of nitrogen gas equal to
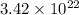 molecules of this substance is, 1.27 liters.
molecules of this substance is, 1.27 liters.