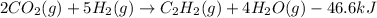Answer:
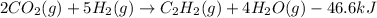
Step-by-step explanation:
As energy is absorbed therefore it is an endothermic reaction. Hence energy value should be written in the product side with a negative sign.
Reaction:
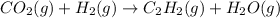
C balance:
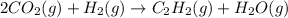
H and O balance:
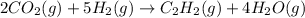
Here 2 moles of
 react. So, energy absorbed during the reaction is
react. So, energy absorbed during the reaction is
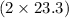 kJ or 46.6 kJ
kJ or 46.6 kJ
Energy balance:
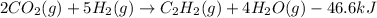
Balanced thermochemical equation: