Hii there !
The reaction accompanying production of water in this case would be -
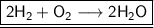
Thus ,by observing the reaction, we can conclude that 2 mole of Hydrogen
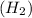 react with 1 mole of oxygen
react with 1 mole of oxygen
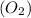 to produce 2 mole of water
to produce 2 mole of water
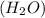
Now,
To produce 1 moles of water.
Moles of Oxygen
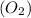 required
required
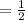 moles
moles
Similarly,
To produce 4 moles of water.
 Moles of Oxygen
Moles of Oxygen
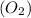 required =
required =
 moles
moles
 2 moles
2 moles
Hope it helps.