Answer:
There are 6 non bonding pair of electrons present in Lewis structure of peroxide ion.
Step-by-step explanation:
Ionic formula of peroxide ion is
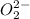
Total number of valence electron present in peroxide ion is 14 (12 electrons come from two oxygen atoms and another 2 electrons come from negative charges).
After fulfilling octet rule, Lewis structure of peroxide shows that each oxygen atom contains three non bonding electron pairs and a covalent bond.
Hence there are 6 non bonding pair of electrons present in Lewis structure of peroxide ion.
Lewis structure has been given below.