Answer : The volume of gas occupy at a pressure of 18 psi is, 520.8 mL
Explanation :
Boyle's Law : It is defined as the pressure of the gas is inversely proportional to the volume of the gas at constant temperature and number of moles.
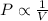
or,

where,
 = initial pressure = 12.5 psi
= initial pressure = 12.5 psi
 = final pressure = 18 psi
= final pressure = 18 psi
 = initial volume = 750 mL
= initial volume = 750 mL
 = final volume = ?
= final volume = ?
Now put all the given values in the above equation, we get:
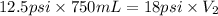
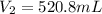
Thus, the volume of gas occupy at a pressure of 18 psi is, 520.8 mL