Answer : The number of moles of oxygen formed are, 0.255 moles.
Explanation :
The given chemical reaction is:
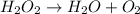
This reaction is an unbalanced chemical reaction because in this reaction number of oxygen atoms are not balanced.
In order to balance the chemical equation, the coefficient '2' put before the
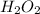 and
and
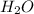 then we get the balanced chemical equation.
then we get the balanced chemical equation.
The balanced chemical reaction will be:
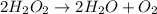
Now we have to calculate the number of moles of oxygen formed.
From the balanced chemical reaction we conclude that,
As, 2 moles of hydrogen peroxide react to give 1 mole of oxygen
So, 0.51 moles of hydrogen peroxide react to give
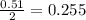 mole of oxygen
mole of oxygen
Therefore, the number of moles of oxygen formed are, 0.255 moles.