The given question is incomplete. The complete question is:
An acidic solution at 25°C will have a hydronium ion concentration ________ and a pH value ________.
![[H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/college/le8croj6vie7g8ce25juw9yh73c1whh6bb.png) >
>
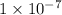 M, pH < 7.00
M, pH < 7.00
![[H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/college/le8croj6vie7g8ce25juw9yh73c1whh6bb.png) <
<
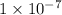 , pH < 7.00
, pH < 7.00
![[H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/college/le8croj6vie7g8ce25juw9yh73c1whh6bb.png) <
<
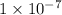 ,, pH > 7.00
,, pH > 7.00
![[H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/college/le8croj6vie7g8ce25juw9yh73c1whh6bb.png) >
>
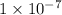 ,, pH > 7.00
,, pH > 7.00
Answer:
![[H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/college/le8croj6vie7g8ce25juw9yh73c1whh6bb.png) >
>
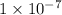 M, pH < 7.00
M, pH < 7.00
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
Acids have pH ranging from 1 to 6.9, bases have pH ranging from 7.1 to 14 and neutral solutions have pH equal to 7.
![pH=-\log [H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/college/8bj6kffduz54mxdb2y3cnu82gbhkta4kix.png)
![7=-log[H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/ajinsgwz2fmulwn6b70qnx95c6ab5mk95g.png)
![[H_3O^+]=10^(-7)](https://img.qammunity.org/2021/formulas/chemistry/high-school/zvgn08prih1kxytu4exlq583paunt9eej3.png)
As pH of acids is less than 7, the hydronium ion concentration is greater than
Thus the correct option is
![[H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/college/le8croj6vie7g8ce25juw9yh73c1whh6bb.png) >
>
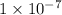 M, pH < 7.00
M, pH < 7.00