Answer:A is SODIUM BICARBONATE,
B is BARIUM HYDROXIDE and
C= HYDROCHLORIC ACID
Step-by-step explanation:
Given Hydrochloric acid, Sodium Carbonate and Barium Chloride.
When A reacts C, a gas is formed. and we know that hydrochloric acid reacts with a carbonate to give a gas.
also Given that A reacts with B to give a white precipitate gives a conclusion that A and B must be a barium hydroxide or Sodium Carbonate therefore means that C must contains HCL
So we are left with Barium hydroxide and Sodium Carbonate which when they react together gives a white precipitate is formed
Reacting Sodium carbonate witrh Hydrochloric acid gives
A + C--- GAS
Molecular equation
Na2co3 (aq) +2HCl(aq)---2NaCl(aq)+ CO(g)+H20 (l)
Net Ionic equation
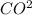 3(aq) +2H+(aq) -- C02(g) +H2O(l)
3(aq) +2H+(aq) -- C02(g) +H2O(l)
When Barium hydroxide reacts with Hydrochloric acis, neutralization occurs and heat is released
B+C---------.> Heat
Molecular Equation
Ba(OH)2 + 2HCl(aq)--- BaCl2 +2H2(l)
Ionic Equation
OH- (aq) +H+(aq)----H20(l)
Therefore A is SODIUM BICARBONATE,
B is BARIUM HYDROXIDE and
C= HYDROCHLORIC ACID