Answer:
a) The relationship at equivalence is that 1 mole of phosphoric acid will need three moles of sodium hydroxide.
b) 0.0035 mole
c) 0.166 M
Step-by-step explanation:
Phosphoric acid is tripotic because it has 3 acidic hydrogen atom surrounding it.
The equation of the reaction is expressed as:

1 mole 3 mole
The relationship at equivalence is that 1 mole of phosphoric acid will need three moles of sodium hydroxide.
b) if 10.00 mL of a phosphoric acid solution required the addition of 17.50 mL of a 0.200 M NaOH(aq) to reach the endpoint; Then the molarity of the solution is calculated as follows

10 ml 17.50 ml
(x) M 0.200 M
Molarity =
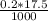
= 0.0035 mole
c) What was the molar concentration of phosphoric acid in the original stock solution?
By stoichiometry, converting moles of NaOH to H₃PO₄; we have
=
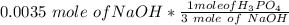
= 0.00166 mole of H₃PO₄
Using the molarity equation to determine the molar concentration of phosphoric acid in the original stock solution; we have:
Molar Concentration =
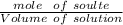
Molar Concentration =
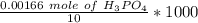
Molar Concentration = 0.166 M
∴ the molar concentration of phosphoric acid in the original stock solution = 0.166 M