0.17 M is the is the molal concentration of this solution
Step-by-step explanation:
Data given:
freezing point of glucose solution = -0.325 degree celsius
molal concentration of the solution =?
solution is of glucose=?
atomic mass of glucose = 180.01 grams/mole
freezing point of glucose = 146 degrees
freezing point of water = 0 degrees
Kf of glucose = 1.86 °C
ΔT = (freezing point of solvent) - (freezing point of solution)
ΔT = 0.325 degree celsius
molality =?
ΔT = Kfm
rearranging the equation:
m =
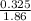
m= 0.17 M
molal concentration of the glucose solution is 0.17 M