Answer:
A. They are equal.
B. The ratio of ions to molecules is 1 to 2.78× 10⁸.
Step-by-step explanation:
A. Concentration of ions
In pure water, [H₃O⁺] = [OH⁻] = 1.00 × 10⁻⁷ mol·L⁻¹.
The concentration of hydroxide ions equals that of hydronium ions.
B. Ratio of ions to molecules
Assume you have 1 L of pure water.
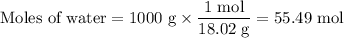
You have 1.00× 10⁻⁷ mol·L⁻¹ [H₃O⁺] and 1.00× 10⁻⁷ mol·L⁻¹ [OH⁻], so you have 2,00 mol·L⁻¹ of ions.
The ratio of concentrations is
![\frac{\text{[Ions]}}{\text{[Molecules]}} = (2.00 * 10^(-7))/(55.49) = ( 3.60 * 10^(-5))/(1) = \mathbf{(1)/(2.78 * 10^(8))}](https://img.qammunity.org/2021/formulas/chemistry/high-school/w9irxar42gbkq1s5el891ls68vsfcc3f5x.png)