Answer:
The magnitude of the net force F₁₂₀ on the lid when the air inside the cooker has been heated to 120 °C is
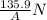
Step-by-step explanation:
Here we have
Initial temperature of air T₁ = 20 °C = 293.15 K
Final temperature of air T₁ = 120 °C = 393.15 K
Initial pressure P₁ = 1 atm = 101325 Pa
Final pressure P₂ = Required
Area = A
Therefore we have for the pressure cooker, the volume is constant that is does not change
By Chales law
P₁/T₁ = P₂/T₂
P₂ = T₂×P₁/T₁ = 393.15 K× (101325 Pa/293.15 K) = 135,889.22 Pa
∴ P₂ = 135.88922 KPa = 135.9 kPa
Where Force =
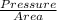 we have
we have
Force =
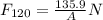 .
.