Answer:
(a) The change in internal energy of the weight lifter is -6.3 × 10⁵ J
(b) The amount of calories of food to be consumed to replace the lost energy is 151.46 calories
Step-by-step explanation:
Here we have
Mass of water, m = 0.200 kg
Latent heat of vaporization, L = 2.42 × 10⁶ J/kg
Work done in lifting the weight = 1.50 × 10⁵ J
(a) Therefore the heat required to vaporize the 0.200 kg of water is
Q = m×L = 0.200 × 2.42 × 10⁶ J/kg = -484,000 J ( -ve as energy is spent)
Therefore, the change in internal energy, ΔU of the weight lifter is;
ΔU = Q - W = -484,000 J - 1.50 × 10⁵ J = -634,000 J = -6.3 × 10⁵ J
(b) The number of calories of food is given by;
1 calorie = 4186 J
Therefore, 6.3 × 10⁵ J contains
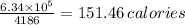
151.46 calories should be consumed to make up for the energy used.