5. 1.16 x
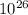 moles moles of pennies would be required to equal the mass of the moon.
moles moles of pennies would be required to equal the mass of the moon.
6. 12.86 moles of ethanol are in a 750 ml bottle of vodka.
Step-by-step explanation:
5 .Data given:
mass of penny = 2.5 grams
atomic mass of penny = 62.93 grams/mole
moles present in mass of the moon given as = 7.3 x
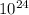 kg
kg
number of moles =

number of moles =
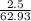
0.039 moles of penny is present in 2.5 grams
0.039 moles of penny in 2.5 grams of it
so, x moles in 7.3 X
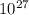 grams
grams
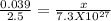
x = 1.16 x
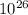 moles
moles
so when the mass of the penny given is equal to the mass of moon, number of moles of penny present is 1.1 x
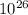 .
.
6.
Given:
vodka = 40% ethanol
volume of vodka bottle = 750 ml
moles of ethanol =?
density of ethanol =0.79 g/ml
atomic mass of ethanol = 46.07 grams/mole
so, from the density of ethanol given we can calculate how much ethanol is present in the solution.
density =

density x volume = mass
0.79 x 750 = 592.5 grams
number of moles =

number of moles of ethanol =
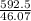
= 12.86 moles of ethanol