Answer:
The balanced molecular equation for the reaction :
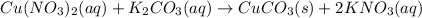
Step-by-step explanation:
The reaction between copper(II) nitrate and potassium carbonate gives solid precipitate of copper(II) carbonate and aqueous solution of potassium nitrate.
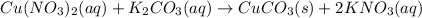
According to reaction, 1 mole of copper(II) nitrate reacts with 1 mole of potassium carbonate to give 1 mole of copper(II) carbonate and 2 moles of potassium nitrate,