Answer : The concentration (in molarity) of the unknown solution Q is, 0.265
Explanation :
Using Beer-Lambert's law :

where,
A = absorbance of solution
C = concentration of solution
l = path length
 = molar absorptivity coefficient
= molar absorptivity coefficient
From the Beer's Law plot between absorbance and concentration we concldue that the slope is equal to
 and path length is 1 cm.
and path length is 1 cm.
As we are given that:
Slope = 0.543 M⁻¹
and,
Slope =

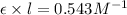
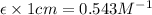
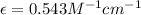
Now we have to determine the concentration (in molarity) of the unknown solution Q.
Using Beer-Lambert's law :

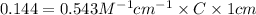
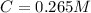
Therefore, the concentration (in molarity) of the unknown solution Q is, 0.265