Answer:
The enthalpy of the dissociation of the magnesium chloride is -168.2 kJ/mol.
Step-by-step explanation:
Mass of magnesium chloride = 0.620 g
Moles of magnesium chloride =
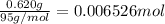
Mass of water = 112.00 g
Mass of solution, m = 0.62 g + 112 g =112.62 g
Heat capacity of the solution = c =
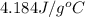
Initial temperature of the solution =
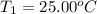
Final temperature of the solution =
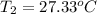
Heat gained by solution = Q
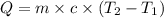
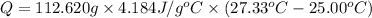
Q = 1,097.90 J =1.09790 kJ ≈ 1.098 kJ
Enthalpy of the dissociation magnesium chloride :
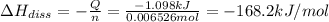
The enthalpy of the dissociation of the magnesium chloride is -168.2 kJ/mol.